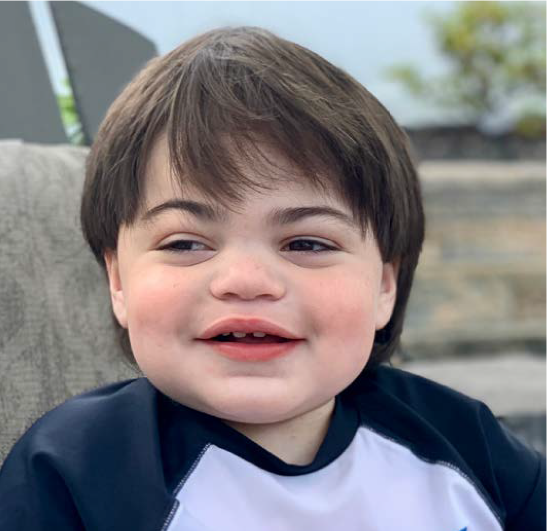
Patient Information
Our current focus is on mucopolysaccharidosis II
(MPS II, Hunter syndrome)
MPS II is a rare condition that affects many parts of the body, occurring in around 1 in 100,000 births and almost exclusively in males


Causes and Symptoms
MPS II is caused by a deficiency of the key enzyme responsible for the first step in the breakdown of glycosaminoglycans (GAGs), causing a progressive accumulation in nearly all cell types, in all tissues and organs. It is possible to address this deficiency using the recombinant enzyme iduronate-2-sulfatase (IDS).
Cognitive function is affected by GAGs building up in the brain, leading to behavioural, emotional, social, and sleep disturbances, as well as learning and memory difficulties. Some common physical symptoms of MPS II include heart and lung disease, hearing loss, enlarged abdomen, coarse facial features and a large head, abnormal dentition, enlarged tongue and tonsils, joint stiffness, skeletal deformities, carpal tunnel syndrome, and short stature.
There is a wide range of cognitive impact, but patients are usually categorised into two groups based on the degree of cognitive ability. Treatments for MPS II should treat both groups.
Life expectancy ranges from the teens for the most severely affected, to the fifties or sixties for those patients with less severe disease.
For more information on living with MPS II visit the MPS Society. A charity providing professional support to individuals and families affected by MPS in the UK.
Current Treatment Options
Currently, the most widely available treatment for most patients is weekly enzyme replacement therapy (ERT) with infusions of recombinant IDS enzyme, which leads to functional improvements and enhances quality of life, even in severe cases of the disease. Because IDS is a large molecule drug that cannot cross the blood-brain barrier, this therapy does not enter the brain and so cannot treat the neurological impairment associated with MPS II.
Our clinical trial is focused on GNR-055, a fusion protein that uses human insulin receptors to cross the blood-brain barrier.